Description
xA&D have been a leading provider of medical devices for both home healthcare and healthcare professionals worldwide since we were established in 1977.
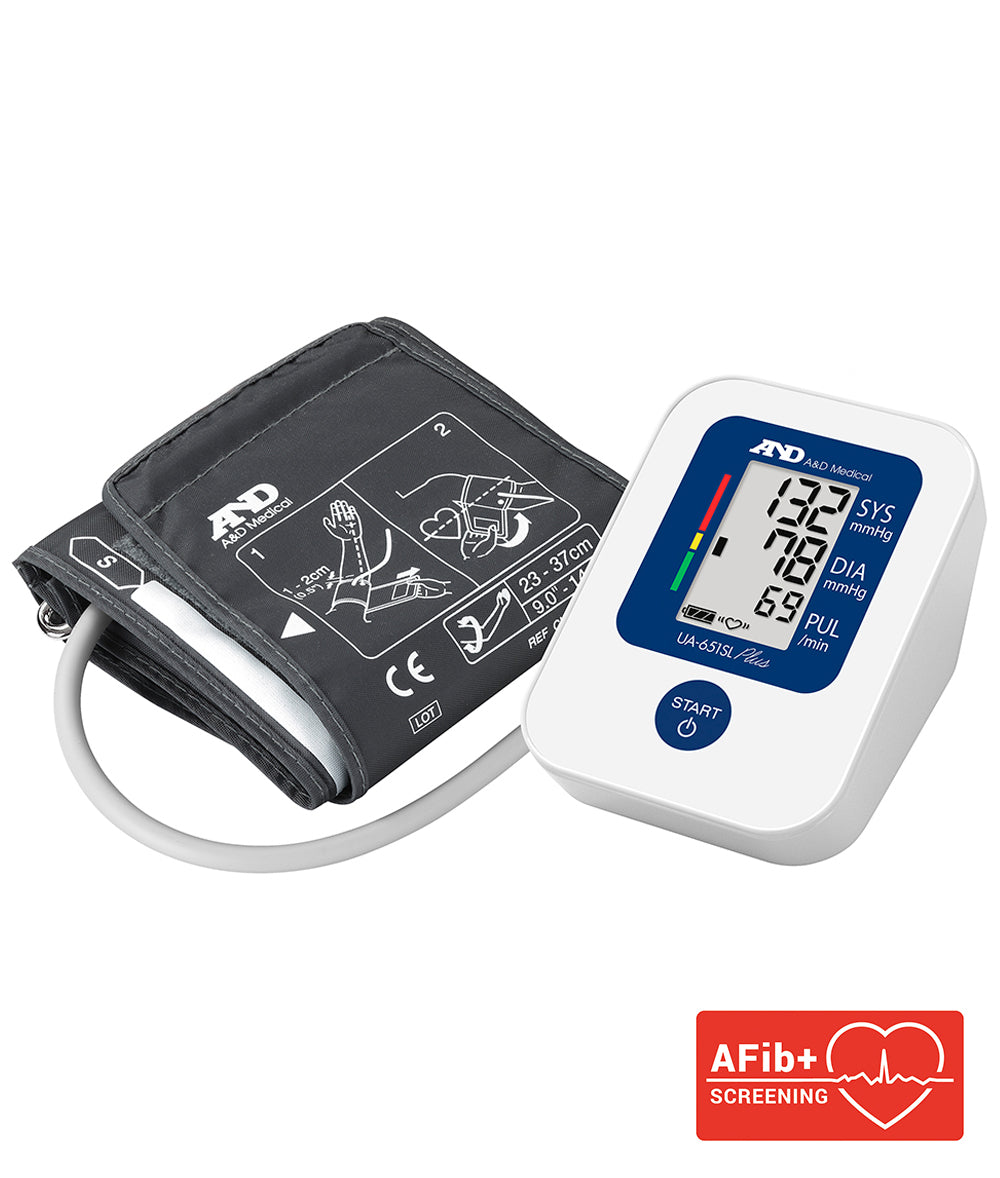
The UA-651SLPlus benefits from many of the features developed by A&D for professional clinical use.
Highlights
- One button control – with latest 3rd generation fully automatic measurement technology
- Unique A&D latex-free and metal-free Cuff (23-37cm)
- AFib+ Screening Indicator
- World Health Organisation (WHO) Blood pressure classification indicator – for easy and clear understanding of Blood Pressure readings
- 60 reading memory function– for recording trending
- Optional multi cuff ranges from 16-45cm
- Average memory reading function
- Clinically validated via the BHS protocol – A/A
- AC Adapter socket and carry case
- Pack contains free batteries
- 5 year warranty – for complete peace of mind (excluding cuff and batteries)
Features
A clear step towards a healthy lifestyle, the monitor benefits from extremely accurate Oscillometric BP measurement, first developed and patented by A&D back in 1984.
The UA-651SLPlus benefits from A&D’s AFib+ technology which screens for IHB and Atrial Fibrillation in a single reading – shown by the IHB/AFib icon. This enables you to see how often it has occurred – if it appears frequently, you should consult your Doctor/GP for further advice.
This IHB/AFib+ function is not to be used for diagnosis, but for monitoring purposes only. IHB (Irregular Heart Beat) technology was first pioneered by A&D in 2001 and the AFib+ screening can now be found across the range of A&D blood pressure monitors.
The monitor also benefits from extremely accurate Oscillometric BP measurement, first developed and patented by A&D back in 1984.
The device also utilizes the latest 3rd generation technology from A&D, with an easy one button operation incorporating the advantage of automatic fuzzy logic controlled inflation.
The UA-651SLPlus is supplied with the Latex and metal free, Cuff (23-37cm) giving the user a more comfortable experience. Additional cuffs can be purchased separately offering a cuff range from 16-45cm.
The UA-651SLPlus is clinically validated to the world recognized European Hypertension Society (ESH) guidelines so the user can have complete confidence in their readings.
The average value feature of the stored data (up to 60 readings) along with the WHO Blood pressure classification indictor allows the user to better control their management program.
The quality of the UA-651SLPlus allows A&D to offer the user a 5 Year warranty (excluding batteries and cuff).
The UA-651SLPlus provides the user a leading cost effective solution with the reassurance of advanced technology and ESH clinical validation
Box Contains:
- UA-651SLPlus Automatic Blood Pressure Monitor
- Latex and Metal Free Cuff (23-37 cm)
- Instruction Manual
- 4 x AAA Batteries
- Choosing a selection results in a full page refresh.


